Current projects
Quantifying Lysosomal pH with mScarlet Lifetime
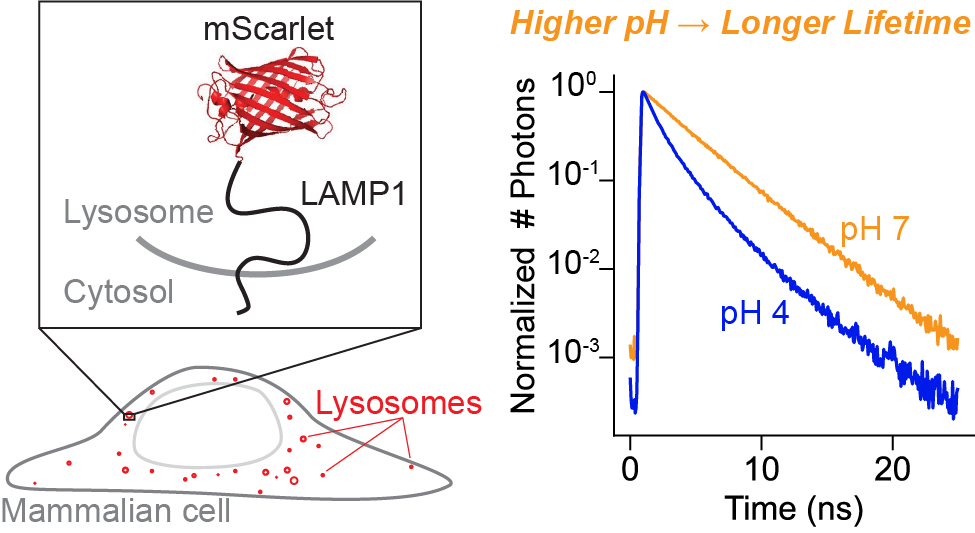
The lysosome maintains a highly acidic pH, which is critical for successful lysosomal catabolism. Lysosomal pH (pHlys) is difficult to measure because of the simultaneous need for a sensor with large dynamic range, genetic targetability, low pKa, and a quantitative readout. To meet this need, we explored the fluorescence lifetime of the published fluorescent protein mScarlet as a quantitative reporter for pH. Using an mScarlet-LAMP1 fusion protein, we are able to record pH at individual lysosomes in cultured cells.
mScarlet lifetime works well to report pH in the range pH 4 to pH 6. This is a great match for the mammalian lysosome, but the tool could in principle be applied much more broadly. You can read more here, and please don't hesitate to contact me if you're interested in trying the technique out yourself!
Ph.D. work
Measuring Membrane Potential Optically
All cells maintain a membrane potential (Vmem), or a voltage across their plasma membrane. Millisecond-timescale changes in Vmem transmit information in excitable cells such as neurons and cardiomyocytes. Resting Vmem is present in all cells and has been implicated in diverse processes ranging from differentiation to body patterning. There is an unmet need for better tools to quantify Vmem so that we can better understand how it functions as a signal in the body. Although patch-clamp electrophysiology is the gold standard for precise Vmem recordings, it is low throughput, highly invasive, and intractable over long timescales.
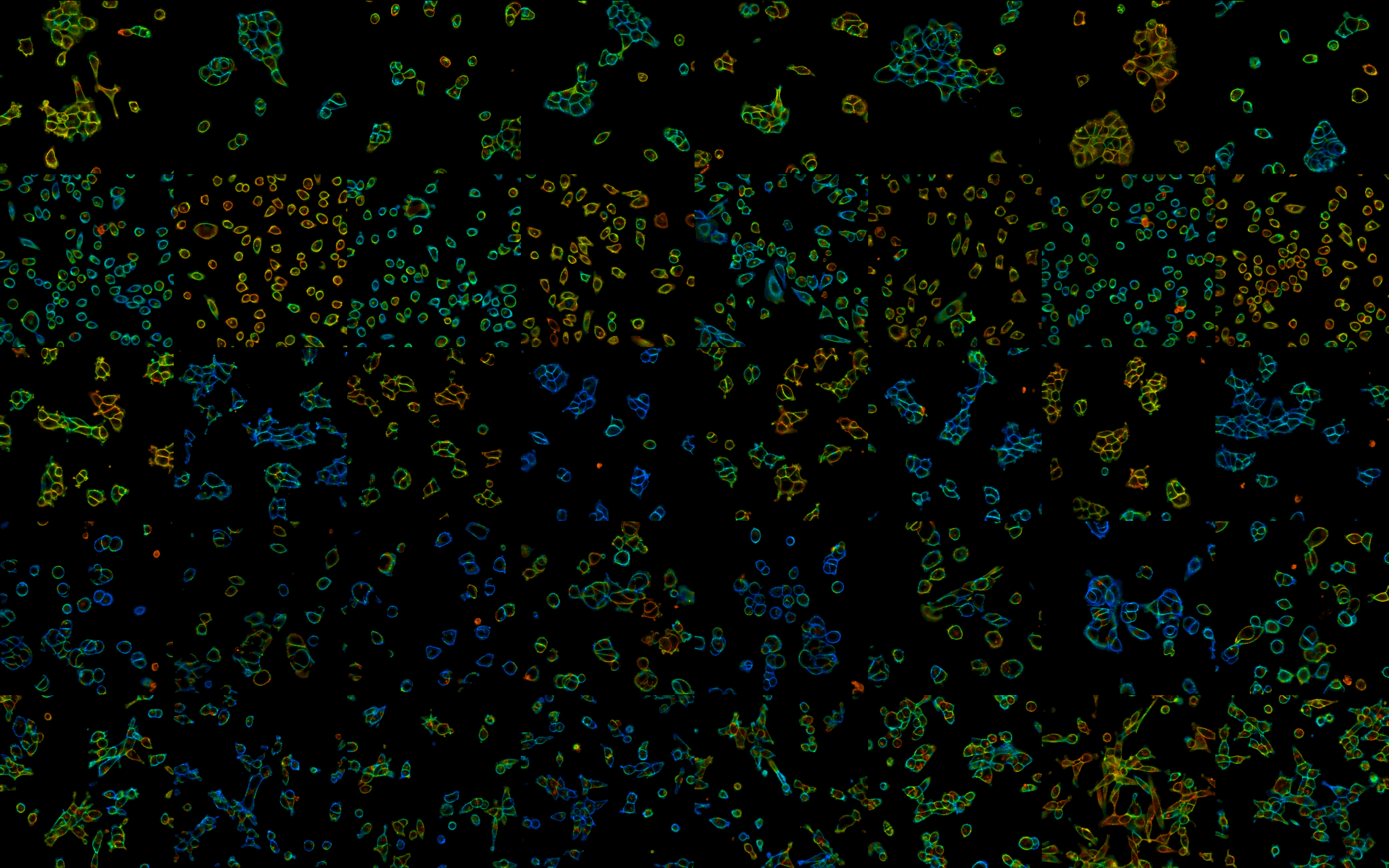
To address this need, we developed and applied an optical method for recording absolute Vmem with fluorescence lifetime imaging (dubbed "VF-FLIM"). VF-FLIM exhibits 100-fold improved throughput over patch-clamp electrophysiology and 20-fold improved voltage resolution over previously reported optical techniques. Using VF-FLIM, I uncovered a Vmem response to epidermal growth factor stimulation in carcinoma cells and identified the ion channel that mediates this signal. You can read more about this work in eLife.
The composite image above is a 5x8 grid of lifetime images, where color represents resting membrane potential of cells under different conditions. It originally appeared as part of the eLife digest describing this work (CC-BY License).
Understanding and Designing Sensors
Fluorescence lifetime imaging can also offer more detailed insight about how fluorescent sensors work, which is super informative if you're trying to develop better ones. In collaboration with some awesome synthetic chemists, I used fluorescence lifetime to better characterize photoinduced electron transfer in VoltageFluor dyes. You can read more about this work in RSC Chemical Biology or on chemRxiv.